
I recently updated Relativistic interpretation of Casimir Effect which establishes the foudation I expand upon here. My premise is that a relativistic interpretation leads to the common underlying environment which is exploited in claims of excess heat by Rossi, Mills, Arata, Haisch, Moddel,
Moller and others going all the way back to Langmuir and his discovery of anomalous heat when welding with hydrogen gas and tungsten electrodes in the 1920′s. These researchers may employ different methods of extracting energy which could even lead to otherwise unfavorable nuclear reactions but it is my position that the initial energy extraction depends upon naturally occurring Heisenberg traps where the constant motion of confined gases with respect to changing Casimir geometry causes changes in energy density. There is one method proposed by Haisch and Moddel that circulates noble gases relative to intentional changes in Casimir geometry that exploits a tiny energy gain based on mechanism they refer to as “Lamb Pinch”. The Haisch and Moddel prototype builds Casimir tunnels segregated by insulation layers to create a synthetic skeletal catalyst. Other claims of anomalous heat are typically based on hydrogen or deuterium and run the gambit from chemical to nuclear either alone or in combination with other mechanisms. My premise for initial energy gain with heated hydrogen gas is that the phenomena we refer to as catalytic disassociation is powered by the constant motion of gas which is in itself a macro example of ZPE based on HUP. The chaotic and random nature of this motion is normally considered un-exploitable however, IMHO, conditions where energy density is caused to vary are not normal and do provide an opportunity to exploit this motion! Normally we know energy density only changes as you ascend or descend by slow gradients through a gravitational well. At the nano scale however we know through Cavity QED that the isotropy of gravity can be suddenly broken by Casimir boundaries. It is easy to see at the macro scale how one could easily gain energy if we had free transportation like gas atoms that transported us between regions with different gravitational levels. We could simply lift items in low gravity and drop them in high gravity, but of course we don’t have free transportation at the macro scale. At the nano scale we know gas atoms can be disassociated at much lower than normal temperatures due to catalysts of Casimir geometry like the skeletal catalyst Rayney nickel used in the Black Light process. It is my premise that molecular gases such as hydrogen oppose these sudden changes in energy density caused by changes in Casimir geometry (referred to as catalytic disassociation) while atomic gases such as h1 are unaffected, explaining molecular disassociation at lower temperatures. This asymmetry between atomic/h1 and molecular/h2 bond states is the basis for a Heisenberg trap – the chemical equivalent of lifting and dropping weights in different gravitational zones, only here, the transportation between zones really is free due to the gas property of constant motion. You still need to initially invest enough heat energy such that the disassociation threshold is within range to be triggered by opposition to change in vacuum energy density. This effectively discounts the heat energy needed to disassociate the molecule but when these atoms reform a molecule the help provided by the constant motion of gas to disassociate molecules does not have a mirror property opposing molecular formation ! The process is not conservative – the opposition to molecular motion discounts the energy associated with molecular disassociation but the energy associated with molecular formation is not affected. The energy released by molecular formation also warms the cavity, thus further reducing our need to add heat on each subsequent cycle. The problem is that as heat builds more and more of the gas population remains disassociated only falling very slowly below the heat threshold to reform molecules ( a kind of self limiting slow over unity). Since we are essentially returning merchandise that we bought at discount for full price we need to accelerate this endless cycle between h1 and h2 WRT the threshold in order to extract any meaningful energy. The Ross E-Catalyzer is thought to accomplish this by extracting the heat with a massive cooling loop to pull the gas population temperature just under the disassociation threshold while using a PWM current pulse delivered into the nickel powder to then drive as much gas as possible back over the disassociation threshold. It remains controversial whether the resistive heating of the E-cat is fast enough to contribute at this speed or if the field generated by the current directly effects the Casimir geometry of the nano powders through magnetostriction to disassociate molecules during the pulsed on time that will cool and reform molecules during the off time. A kind of sweet spot must be balanced between heat extraction that maximizes the number of ”still warm” molecules available for disassociation and the number of these molecules that can be disassociated by PWM current to the resistive heaters during the ”on” portion of the heater waveform.
It seems counter-intuitive but instead of just throttling back this type of reaction we MUST remove heat, not only to store the energy gain but we have to cool the disassociated atoms enough that nature takes over and they reform molecules allowing us to repeat the cycle over and over again. The random motion of gas relative to Casimir geometry changes the energy density being experienced by the gas molecules. Atoms are simply reoriented by this change in energy density but those atoms sharing covalent bonds (molecules) are held in the same orientation they posessed when the molecule formed by the covalent bond. This “pressure” the covalent bond feels when energy density changes discounts the energy needed to disassoiate the molecule such that it can occur at a much lower temperature – when these atoms later re-form a new molecule they release the full energy associated with hydrogen atoms dropping to the lower molecular energy state including even the energy contributed in the previous cycle from the combination of gas motion and change in energy density. We are getting a full refund for a discounted purchase.
The Relativistic interpretation of Casimir effect proposes that the reduction in vacuum energy density between Casimir plates exactly mirrors the relativistic effects we observe when energy density increases due to acceleration or gravity. We see the effect of increased energy density as slowing time in the Twins Paradox where the dilation and contraction can only be determined by relative measure. I am proposing these properties are reflected when vacuum energy density is lowered such that time is accelerated – contraction remains absolute in either type of dilation but with spatial velocity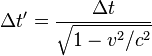
you have a vector between space and time where velocity slowly reduces past .7C and the contraction appears along the dimension of travel. When energy density is instead reduced by the supression mechanism of Casimir geometry the rate of C is directly manipulated! you have no need for spatial displacement and the contraction is symetrical along the time axis, seeming to contract equally from any spatial axis. I think this accounts for many of the claimed forms of condensed gas such as hydrino , fractional hydrogen, deuterium ice and the like. A mathematical relationship between the formula for Casimir force and time dilation due to velocity should allow us to solve for time dilation as a function of the plate Area “A” over plate seperation “a”^4 . I think Casimir geometry can be broken down into an array of equivalent accelerations and t’ based on the mass of h1 or h2 . Like Peng Chen at Cornell discovered with respect to nano tubes you need “changes” in geometry to create catalytic action – therefore the most irregularities at the smallest Casimir geometry makes the best catalyst by presenting the most dynamic array of force levels. These rapid changes in equivalent acceleration are hard to notice from our perspective outside the cavity where the atoms appear to become slower, smaller, condensed or “fractional” to us using a microscope from our inertial frame.
I am only just starting the math – the equations below are notyet in the best forms for use – I am still seeking better forms but my premise is that there is a relationship between the Gamma and Casimir formula [ t’ and A/a^4 ] and that gas reactants circulating between Casimir boundaries experience an array of t’ values – Always accelerated from our perspective but more so and less so relative to changes in geometry. What we perceive as catalytic action from our perspective outside the Casimir boundaries are very real changes in acceleration vectors for gas inside the boundaries.
The Casimir force per unit area Fc / A for idealized, perfectly conducting plates with vacuum between them is
where
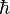 (hbar, ħ) is the reduced Planck constant,
(hbar, ħ) is the reduced Planck constant,- c is the speed of light,
- a is the distance between the two plates.
- ————————————————————-
- Time dilation due to spatial velocity
-

You update is very interesting. Since I last commented on your theory I would like to draw your attention to the following.
A group led by professor Per Moller at DTU in Denmark have covered an electrode with a homogeneous coating of Renay Nickle which then has been permamently embedded on the electrode. This is something which until now was considered impossible. They have used the Renay Nickle covered electrode the seize of half a A4 page to be part of a pilot plant used to in dissociation process of water to hydrogen and Oxygen. The plant produces 7 m3 of hydrogen pr hour. Their setup has been running since end of July 2010 without any deteriation of the electrode in fact I have been informed it is running more efficient than ever. Their process running in a water bath at
Even though they have not made public how they have embedded the Renay Nickle due to pending patent application they have informed that process takes place at a temperature around 800 – 900 degrees Celsius. During the process all the aluminium is removed from the Renay Nickle leaving the electrode covered with a very fine 3 dimensional net of Nickle. If there is a c ontribution it would go a way to prove your theory.
The electrolytic process takes place at a temperature of 100 degrees celcius and the only thing that is added during the process is pure water.
I have asked if they have measure the temperature at the electrode covered with the embedded Renay Nickle, this has not been done yet, however I believe that there might be a positive contribution to the temperature during while the process is running. The group has said that they will measure and publish if there is a positive contribution of heat during the process.
I believe that the electrode they have made at DTU lends itself to be used in a controlled experiment similar to ones published by the group headed by Professor Jansson at Rowan University. It should be so much easier to use the DTU electrode than the powder that is now used.
I have attached the Danish article that tells about the process they have developed at DTU. It should be noted the correct amount of Hydrogen produced is 7m3 and not 4 m3 as stated in the published article. The article can be translated using the Google Translating technique.
________________________________________
Ny produktionsmetode muliggør ‘stinkende billig’ brint
Med avanceret procesteknik er det lykkes forskere på DTU at udvikle nye elektroder til katalytisk fremstilling af brint helt uden brug af de ædle metaller, som ellers kræves for at kunne holde en høj virkningsgrad.
Klik på grafikken for at se den i fuld størrelse.
________________________________________
________________________________________
Af Bjørn Godske, Sanne Wittrup, fredag 10. dec 2010 kl. 06:03
Forskere ved DTU er nu i stand til at løfte effektiviteten af brintfremstilling ved hjælp af elektrolyse til et helt nyt niveau, uden at det går ud over omkostningerne. Det skyldes udvikling af en proces til fremstilling af elektroder.
Materialerne og grundideen til de nye elektroder har egentlig været kendt i mange år:
»Men vi har bare fremstillet dem en hel del smartere, end andre har været i stand til,« siger professor Per Møller, der sammen med ph.d.- studerende Cecilia Kristin Kjartansdóttir, har stået i spidsen for udviklingsarbejdet, som er foregået på afdelingen for Materialeteknologi og Udvikling under Institut for Mekanisk Teknologi
Ideen er, at elektroderne er påført et tyndt lag af såkaldt ‘Raney-nikkel’ – en legering af nikkel og aluminium først beskrevet for over 90 år siden:
»Ved hjælp af elektrokemiske processer er vi i stand til selektivt at fjerne aluminium og dermed opbygge en ekstremt stor overflade, hvor den katalytiske proces kan foregå,« forklarer Per Møller.
Det vil med andre ord sige, at forskerne helt undgår at bruge det kostbare platin, som ellers har været nødvendigt for at holde virkningsgraden oppe:
»Det er derfor, vi kan gøre det stinkende billigt. Vi skal ikke ud at opfinde nye materialer eller metoder. Det hele er principielt kendt. Det eneste, vi har tilført, er viden om elektrokemisk procesteknologi,« siger Per Møller.
Og netop derfor vil Per Møller heller ikke gå mere i detaljer omkring, hvordan forskerne helt præcist har gjort, for det er her hemmeligheden ligger. Resultatet er en elektrode, som koster under en tiendedel af, hvad man ellers skal betale.
Erhvervssamarbejde i Herning
Arbejdet med udvikling af nye metoder til katalytisk fremstilling af brint blev for tre år siden sat i gang af virksomheden GreenHydrogen i Herning. Man valgte at satse på to forskellige metoder: Keramiske elektroder, som Risø DTU i Roskilde har specialiseret sig i, og Raney-nikkel-elektroderne fra Per Møllers forskningsgruppe i Lundtofte. Direktør i GreenHydrogen Jørgen Krogsgaard Jensen siger:
»Vi har med DTU’s forskning fået vist, at der kan opnås betydelige kvantespring på virkningsgraden. Vi har hele tiden fokuseret på, at der skal udvikles kommercielle anlæg, og det ser vi ud til at kunne opnå,« siger Jørgen Krogsgaard Jensen.
Lige nu står et anlæg og kører hos H2-College i Herning. Det producerer 4 normalkubikmeter brint i timen ved 30 bar og en procestemperatur på 100 grader celsius. Det eneste, som skal tilføres, er rent vand og el.
Den store udfordring for GreenHydrogen bliver nu at bære resultaterne fra laboratoriet over i anlægget, og Jørgen Krogsgaard Jensen forventer, at de inden længe vil have et anlæg, som kan producerer én normalkubikmeter brint ud fra 4 kWh.
Prisen for den producerede brint, afhænger af et væld af faktorer, men Jørgen Krogsgaard Jensen vurderer, at med de resultater, man allerede i dag kan fremvise, så ligger de samlede produktionsomkostninger pr. kilo brint på cirka 60 procent af eksisterende metoder.
Det kan blive endnu bedre
Test af de nye brintelektroder viser, at virkningsgraden i laboratoriet kommer over 90 procent, uden at der opstår slid på elektroderne. Og Per Møller tror, at den kan blive endnu bedre:
»Indtil nu har vi kun koncentreret os om katodesiden, altså der hvor brinten produceres. Næste skridt bliver at optimere anodesiden af processen, altså der hvor ilten produceres,« siger han og forventer, at virkningsgraden kan få endnu et nøk opad.
Nu er det planen, at 2011 og 2012 skal bruges på yderligere udvikling og test hos udvalgte kunder. Jørgen Krogsgaard Jensen forventer, at de første kommercielle anlæg vil være klar i 2013.
Response to comment by Chairman Srinavasan of ICCF16 :
Many years ago, we met at Cold Fusion Conferences. Since then, I have been working in other areas of physics. However, the Italian Cold Fusion experiments rekindled my interests in cold fuison. See http://www.prlog.org/11277508-qed-induced-cold-fusion-in-italy.html
The secret catalyst in the E-Cat proposed by Rossi et al is nothing more than nickel nanoparticles that transmute under QED induced radiation to copper and release nuclear energy – not chemical energy.
Hence, your statement: “Our dream is to have a small fusion power generator or pack with a capacity to produce 20-100 KW of energy in each house. Mankind needs a new source of energy and this could be a major source to meet the ever-increasing demand for power” is therefore unequivocally realizable. See http://blog.newenergytimes.com/2011/02/04/iccf-16-chairman-srinivasan-in-times-of-india/
But there are dangers with the E-Cat that you must overcome. If the nickel nanopartticles are replaced by fissile uranium or plutonium, explosions mayoccur and release radiation. Unless you can control all fissile materials from the face of the earth, you cannot realize your dream. But this may be necessary in the future anyway irrespective of what controls are placed on the E-Cat. Unfortunately, the advantage of a small fusion power generator in every house comes with a risk. But mankind may justify the risks of E-Cat in the future.
Hi Thomas,
there is some recent evidence that the catalyst may be Tungsten powder see below my reply on vortex:
from froarty:
Everyone knows tungsten has been a prime suspect since the days of Langmuir and the atomic welder, As I recall from recent thread, Bohr convinced Langmuir to downplay the anomalous heat in favor of the greatly improved welding temps. I have gotten many messages from a tungsten proponent with a screen name of Lebricahn asking me to give heavier consideration to tungsten powder as a catalyst – the idea being that the tungsten electrodes of Langmuir would function magnitudes better with the increased surface area and geometry over Langmuir’s electrodes. I suspect freshly milled tungsten powder in an oxygen free glove box would be even better. If the tungsten powder surfaces are not allowed to tarnish they should remain much more reactive once any shielding gas is displaced by the working gas. This goes back to a ZPE interpretation of pyrophoricity where the “pyro” path is eliminated allowing the reaction to scale to much higher temperatures based on geometry of both the powder and the working gas. Pyrophoricity is already a rare phenomenon in nature so raising it to an even higher level might explain the difficulty in repeatability.
Fran
From: [email protected] [mailto:[email protected]] On Behalf Of Alain Sepeda
Sent: Thursday, March 01, 2012 1:56 AM
To: [email protected]
Subject: EXTERNAL: Re: [Vo]:Tungsten?
yes it is ited in many larsen slkides as one of the key experiment.
tungsten was seen vaporized in an unusual way when LENR signas were seen.
http://newenergytimes.com/v2/sr/WL/slides/2009June25LatticeEnergySlides.pdf
eg page 59, but many other (including exploding wires)
2012/2/29 Mark Goldes
4) If I remember correctly, Tungsten has been used in other cold fusion systems.
Interesting thought but checkout the spectrum for Ni -> http://www.xrfresearch.com/component/content/article/71-periodic-table/160-xrf-spectrum-nickel.html
Colin
On Wed, Feb 29, 2012 at 8:51 AM, Mark Goldes wrote:
I’ve copied this from ecat news.
A very interesting Comment on the e-catworld.com Blog from a user called “Fluffy”.
It’s about the secret element used in rossi’s e-cat (and maybe in defkalions hyperion).
He thought it’s Tungsten (Wolfram) -> http://en.wikipedia.org/wiki/Tungsten
“Rossi’s Possible Tungsten Line at 8.31 keV
In Andrea Rossi’s original patent application
http://www.wipo.int/patentscope/search/en/detailPdf.jsf?ia=IT2008000532&docIdPdf=id00000009056757&name=%28WO2009125444%29METHOD%20AND%20APPARATUS%20FOR%20CARRYING%20OUT%20NICKEL%20AND%20HYDROGEN%20EXOTHERMAL%20REACTIONS&woNum=WO2009125444&prevRecNum=1&nextRecNum=2&recNum=1&queryString=&office=&sortOption=&prevFilter=&maxRec=
there are two charts that show the results of an XRF (X-ray fluorescence)spectrum analysis on a sample of used powder from an E-Cat unit that had been in operation for an undisclosed period of time. Although many of the elements found in the analysis are labeled on the chart, one significant “spike” or “line” is not. This anomalous line could possibly be the element tungsten.
XRF fluorescence works by subjecting the material to be tested to x-rays, that can knock electrons out of their orbit in the atoms of the sample material. When another electron moves in to fill the gap produced by the missing electron, a photon is emitted. By measuring the energy of these photons (in keV or kilo-electron volts) and how many are produced, you can determine the composition of a sample of material. A chart produced using the data from an XRF spectrum analysis will show a spike or line for each element present. When there is very little of an element in a sample of material these spikes will be small, and perhaps hard to distinguish from “noise” or other elements. However, when there is a lot of a specific element in a sample, the spike or line will have a significant amplitude.
The two charts in Rossi’s patent show many lines, some of which indicate a very significant amount of certain elements. All of the lines that seem to be significant are labeled, except one. If you look at the following chart from his patent you will see that there is one line that is not labeled. This line is between the lines of Nickel and Zinc, and it sits at about 8.3 electron volts.
There have been a few comments on the web about this graph. The following is from the comments section in a story posted on ecatnews.com.
http://ecatnews.com/?p=829
“I went back and counted pixels with MS Paint to do a more thorough job of identifying this component.
It’s not Copper at all. It’s Tungsten.
The material is a Ni-W-Zn alloy metal foam.”
There are also comments on various websites about how Tungsten can behave like a catalyst, and is used in atomic hydrogen torches to separate molecular hydrogen into atomic hydrogen. I remember Rossi stating on his blog that Tungsten is not used in the E-Cat, when asked a question about it. However, after searching his blog at the Journal of Nuclear Physics, I cannot find that comment.
To try and figure out if the anomalous line in this chart could be Tungsten, I did some digging on the internet. As a non-scientist I did not understand everything. However, I did find out that Tungsten has a keV signature that is close to the 8.3.
According to a chart on this website http://www.xrfresearch.com/technology/xrf-spectra/182-xrf-spectrum-tungsten.html, one of Tungsten’s possible signatures is 8.39 keV. This is close to 8.31, which is what I calculated by studying the chart from Rossi’s patent. Also, I found a few references to Tungsten having a signature of 8.3 keV.
It seems like the line between nickel and zinc in the chart could be Tungsten. There are other possibilities, including copper and nickel. However, if that line was copper or nickel, I wonder why it was not labeled? It does not make sense to me that they would not label the line as copper or nickel, if that was the identity of the element. What would make sense to me, is if the element was Tungsten, and they did not label it as such to try and hide the fact Tungsten is used in the powder.
So if this line is Tungsten, how does it fit into what we know about what we have been told about Rossi’s catalyst?
1) Tungsten is not a precious metal. This fits what we have been told, that no precious metals are used in the E-Cat.
2) It has a very high melting point at 3422C which is much higher than the melting point of nickel which is around 1400C. Since we have been told the temperature inside the E-Cat reactor core routinely reaches 1600C, perhaps the addition of Tungsten increases the melting point of the powder inside the E-Cat. Something needs to increase the melting point, because when the nickel melts inside of an E-Cat the reaction sites are destroyed, and the nuclear reactions end. A blend of nickel and Tungsten could be what allows for the E-Cat to operate at higher temperature than the melting point of nickel.
3) We also know that tungsten can be a catalyst in various applications, including helping to produce atomic hydrogen. We have been told that the catalysts in Rossi’s system help produce atomic hydrogen, which can then interact with the reaction sites on the nickel powder. Could tungsten produce atomic hydrogen in an E-Cat?
4) If I remember correctly, Tungsten has been used in other cold fusion systems.
Rossi made an interesting comment on his blog. He stated the following.
Andrea Rossi
February 23rd, 2012 at 11:05 AM
Dear Helmut H.:
Not only MIT, but many others. Many copies derived from our patent application have been made, this is why the competition is on the market: who will be able to produce the best at the best price will win.
Warm Regards,
A.R.
If copies can be derived from the patent application, does that mean that it might have information about the catalysts?
I am hoping that there are people who read E-Catworld that understand more than I do about physics, that can determine the identity of the anomalous line. If we can determine what it is and if tungsten really is used in the E-Cat, it could help us better understand this amazing technology.”
Mark Goldes
Please see below an update on the progress madewith the elektrodes developed by Per Moller in collaboration with Green HydrogenSiemens project could pave the way for hydrogen storage of wind energy
Over the next three years, the new low-cost hydrogen technologies are tested in a collaboration between Siemens and DTU and the company Green Hydrogen. The goal is components for large electrolysis plant that could potentially be used to store wind energy in the form of hydrogen.
Electrolysis and production of hydrogen can take place both locally and centrally. At a central multi-station could partly imagine that hydrogen is used to generate electricity in a fuel cell and partly for the production of synthetic fuels such as methanol and natural gas by various chemical processes. It requires the addition of carbon – for example from gasified biomass
By Bjorn Godske, Monday 28th November 2011 pm. 10:47
With funding from the Foundation’s Siemens has gone directly into the development of alkaline electrolysis based on completely new surface technology developed at DTU.
Over the next three years, the project ‘High-efficiency, low-cost electrode Surfaces for the next generation Alkaline Electrolysis’ develop a technology where the costly precious metals in the electrodes are replaced by so-called Raney nickel without sacrificing efficiency. This whole process can be so cheap that it will be interesting to look at the technology on a large scale.
Siemens has previously to engineer confirmed that looking at suitable storage technologies for wind power in the range of 100 MW, but that you have not committed themselves to a simple technology. Something tells me that electrolysis hydrogen is with the Siemens’ strategic palette.
The efficiency is over 90 percent
The process was developed in collaboration between DTU Mechanical HIRC v / IBT University of Aarhus and Herning-company Green Hydrogen, with the support of EDDP. Jorgen Krogsgaard Jensen, director of Green Hydrogen, says:
“Now we have started to test the concept, it can really scale up what is the reliability and keeps the economy? We are looking, among other things, how much we have to make individual cells and stacks, as it has great significance for what is best technically and in relation to future customers, “says Mr Krogsgaard.
The surfaces produced by a Danish subcontractor, and Jorgen Krogsgaard Jensen highlights the close collaboration between DTU, subcontractor and Green Hydrogen as essential for an effective development process.
As the engineer for a little over a year since the first time described the process (Engineer No. 49/2010), laboratory tests showed that the efficiency was over 90 percent, according to Professor Per Moller from the Department of Mechanics, is a little farther up. While there have been developments in materials:
“We are about to make the manufacturing process of the special Raney-nickel even cheaper, and we hope it can be industrialized as soon as possible – at least as a first electrode catalyst coating,” says Per Moller.
No comments from Siemens
From Siemens’ side has declined to elaborate on how the results from the Danish electrolysis project will feed into a future business strategy. Communications for Siemens Denmark, John Finnich Pedersen says to ing.dk that Siemens has several hydrogen projects underway, and that you will first evaluate results from the project before we publish further plans.
Foundation has contributed 12 million. crowns out of a total project budget of 23.5 million. crowns.